+86-13928321129
jiahewell@jhzhb.com
- All
- Product Name
- Product Keyword
- Product Model
- Product Summary
- Product Description
- Multi Field Search
 English
English English
English
1. Qualification verification
· Medical device certification: confirm whether it holds FDA 510 (k), CE, ISO13485 and other qualifications (such as Jiahewell has passed FDA and ISO13485 certification)
· Material test report: SGS or third-party test certificate for medical-grade silicone gel is required (Jiahewell provides test reports for each batch)
2. Product performance evaluation
Evaluation Dimension | Quality Standard | Jiahewell Parameter |
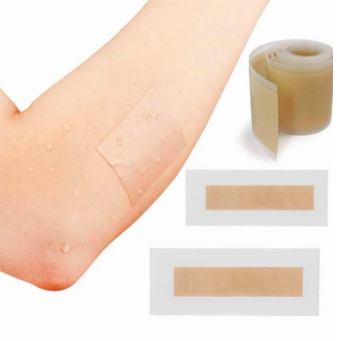
Silicone Gel Purity | ≥99.5% | Medical Grade 99.7% |
Breathability | Micropore density > 500/cm² | 0.01-0.03mm Breathable Structure |
Peel Strength | ≤1.5N/cm (gentle and non-damaging) | 1.2N/cm |
Transmittance | ≥80% | Natural Skin Color Fusion 85% |
3. Production capacity inspection
1. Production capacity scale: Annual production capacity must meet order demand (Jiahewell's own factory has an annual production capacity of 5 million pieces)
2. Quality control process: Is there 10 10,000-level cleanroom and full-process quality inspection (Jiahewell uses AI visual inspection)
3. Customization capability: support OEM/ODM (Jiahewell provides personalized services with a minimum order of 5,000 pieces)
4. Service system verification
·Sample policy: provide free test samples (Jiahewell supports 7-day proofing)
·After-sales response: technical support within 48 hours (Jiahewell provides bilingual services in Chinese and English)
·Case endorsement: require proof of cooperation with 200+ medical institutions (Jiahewell serves 300+ customers worldwide)
5. Market reputation research
1. Third-party evaluation: check the ratings of platforms such as Google Business and Trustpilot
2. Industry ranking: confirm whether it has entered the Global Silicone Scar Products Top 10
3. Patent technology: check whether it has core patents (Jiahewell holds 3 national invention patents)